Statement on THC levels in food
The recall of hemp protein powder (Veganz brand, April 2016) shows that
the term tetrahydrocannabinol (THC) is used imprecisely and reporting is undifferentiated. Limit values are mentioned, although only recommendations are available, which in turn are based on the content of total THC or the content of delta-9-THC. In the present case, the analytically determined total THC value for the assessment of a hemp foodstuff is confused with the EFSA recommendation for delta-9-THC. Thus, independent values are set in relation to each other. General recommendations for the intake of pure THC per kilogram of body weight are wrongly transferred to the guideline values for the intake of total THC through edible foods.
The term “THC”
The term “THC” is often used without differentiating between total THC and delta-9-THC. The total THC content is made up of the psychoactive delta-9-THC, which may actually be present in the food, and the non-psychoactive precursor delta-9-tetrahydrocannabinolic acid (THC-A), which is converted into delta-9-THC during the analytical THC determination. In fresh hemp plants and on the outer shell of hemp seeds, THC-A accounts for up to 90 percent.
However, the total THC content after its analytical determination is also shown as the delta-9-THC content, although the psychoactive substance delta-9-THC in the analyzed food is actually quantitatively well below the analytically determined total delta-9-THC content. This also applies to the THC value information on “lebensmittelwarnung.de” (attachment). For further information on THC analysis, see “Analytical method for THC determination” (see page 3f.)
Assessment of THC in a global context
To date, there are no binding limits for total THC or delta-9-THC in food and its daily intake per kilogram of body weight, neither in the EU nor in Germany. The WHO has also not yet set corresponding values.
A toxicological limit value is defined via the acute reference dose (ARfD) for substances which, due to their acute toxicity, can cause damage to health even after a single or short-term exposure. This is to be distinguished from the toxicological limit for the Acceptable Daily Intake (ADI).
The ADI value refers to the chronic effect of a substance and describes the amount of a substance that a consumer can take in daily and for a lifetime without any recognizable health risk. The definition and calculation of the values are internationally harmonized.
In July 2000, the Federal Institute for Risk Assessment (BfR, formerly BgVV) published an ADI value of 1 to 2 micrograms [1] per kilogram of body weight, taking into account an uncertainty factor of 20 to 40 [2] for total THC on the basis of a minimum active dose of 2.5 milligrams per day and per person (male, 70 kilograms). The BfR thus assumed a chronic THC effect. Based on this calculation, the BfR issued guideline values for THC in food for Germany in 2000 and confirmed them again in 2006.
Guideline values are non-binding recommendations for which minor exceedances are generally tolerated. Products are to be assessed as impaired if the guideline value is exceeded by more than double. [3] The European Food Safety Authority (EFSA) took a different approach in a scientific assessment on “Risks to human health from THC in milk and other foods of animal origin” in 2015, which for the first time recommended an acute reference dose (ARfD) of one microgram of pure delta-9-THC per kilogram of body weight for the same active dose as the BfR. The uncertainty factor of 40 and the setting of an ARfD were justified by observable effects on the central nervous system (mood swings, sedation) and measurement of an increased heart rate within a short time after administration of delta-9-THC. [4] This value of 1 microgram of delta-9-THC per kilogram of body weight has now been applied to the hemp protein powder in the current case when assessing food safety.
The following table illustrates the different reference basis and differing uncertainty factors used by EFSA and BfR to determine the maximum THC intake per day: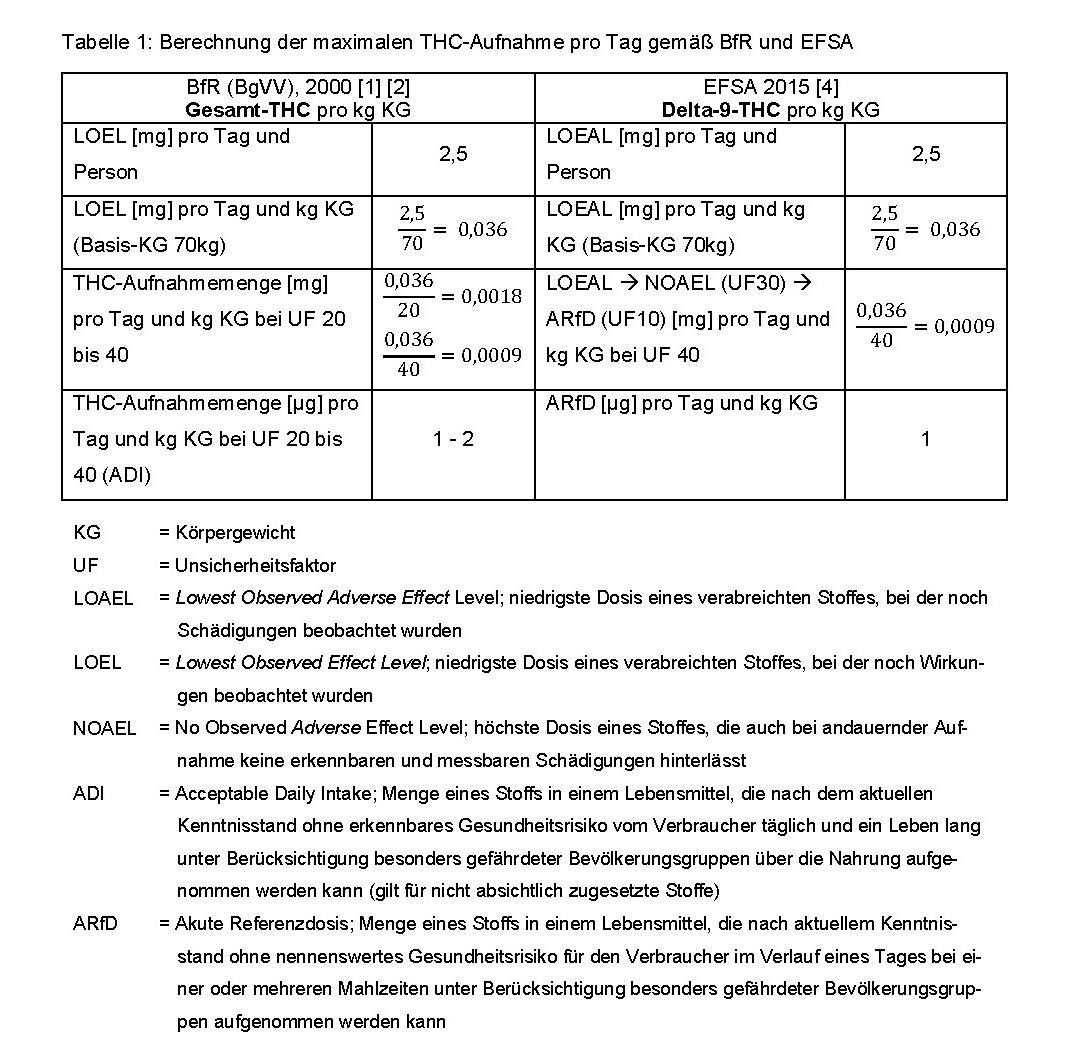
The inconsistent assessment of THC is also evident when comparing national regulations. In contrast to Germany and Europe, Switzerland has defined clear limits for delta-9-THC in food, which are significantly higher than the BfR guideline values. [5] The maximum delta-9-THC value of ten milligrams per kilogram for hemp food raw materials applicable in Canada (the largest producer of hemp food raw materials) is also significantly higher. [6] Analytical method for THC determination
The official (validated) method for THC determination in hemp oil is gas chromatography (GC) [7] in combination with mass spectrometry (MS) or flame ionization detection (FID). [3] [4] The analysis of hemp-containing foodstuffs to determine their total THC content is also carried out by the German authorities using the GC-MS method as standard.
Heat is considered a significant factor influencing the conversion of the non-psychoactive THC-A into the psychoactive delta-9-THC. Consequently, only the total THC content is determined when using the GC-MS method, in which temperatures of 260 to 300 degrees Celsius are used [7]. As already mentioned at the beginning, this total THC content is also shown as the delta-9-THC value. Although only delta-9-THC is present after analyzing the sample, this value is made up of the actual delta-9-THC content and the delta-9-THC content resulting from the conversion of THC-A. Consequently, when determining THC in food, the actual delta-9-THC content in the food concerned is likely to be significantly lower than the analytically determined total THC content due to the method used.
In its 2015 report, the EFSA also explicitly points out that both methods (GC-MS and GC-FID) provide inadequate results with regard to the differentiation of delta-9-THC and THC-A and therefore show a higher delta-9 content than high-performance liquid chromatography (HPLC). Since the temperatures are lower in HPLC analysis, the delta-9 values determined are likely to be significantly closer to the actual delta-9 values of the hemp product analyzed. [4] The use of the GC-MS method, including the total THC content determined with it, is justified by the possibility that THC-A could also be converted into delta-9-THC during heat treatment of the food. [3] In vivo, there is no conversion of THC-A into delta-9-THC. [4] Main criticism of the risk assessment: application of incorrect reference values
The main point of criticism of the risk assessment of hemp foodstuffs is the reference to different reference values (total THC versus delta-9-THC), which are decisive for the assessment of the marketability of a foodstuff by calculating the percentage of THC utilization of the ARfD.
The delta-9-THC content in terms of the total THC content was also determined for Veganz brand hemp protein powder using the GC-MS method. When assessing the marketability of the hemp protein powder, the total THC content determined was then related to the ARfD for delta-9-THC. This leads to an incorrect assessment of marketability, as shown in the following example calculation for the total THC content of 20 micrograms in 25 grams of hemp protein powder (0.8 micrograms per 1 gram of hemp protein powder) determined in the current case:
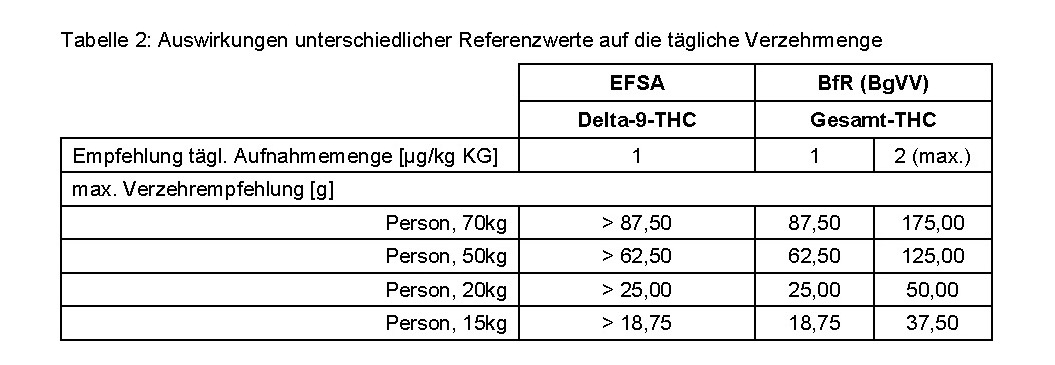
It must therefore be ensured that the correct values are always compared with each other in each evaluation. When referring to the EFSA recommendation, this is the delta-9-THC content (most accurate method: HPLC), when comparing with the BfR recommendation, the total THC content (GC-MS)..
Evaluation of the THC content in ready-to-eat food is missing
The EFSA value refers solely to the delta-9-THC intake per kilogram of body weight, independent of the edible food. EFSA does not make any recommendations on the delta-9-THC content in ready-to-eat food. [4] Therefore, the assessment of the hemp protein powder is wrongly based on the likewise “incorrect” THC value for the ingredient alone, but not for the edible food.
Typically, hemp protein powder is stirred into juices, milkshakes and smoothies and consumed cold, so that a conversion of THC-A into delta-9-THC through the effect of heat can be ruled out. According to the BfR guideline, such hemp protein powder mix drinks fall under the category “all other foods” [1] as prepared, ready-to-eat foods. A total THC guideline value of 0.15 milligrams per kilogram of edible food applies to this product group. [1] The EFSA does not provide any information on the THC content in edible foodstuffs. The recommendation of the European Industrial Hemp Association (EIHA) is 3.5 milligrams per kilogram for an edible food prepared with hemp protein powder. [2] The calculation depends on the composition of the edible food (quantity ratio of the ingredients).
Inconsistency in determining the degree of utilization
A key factor in the assessment of food safety is the utilization rate in
percent of the ARfD. However, a utilization rate of over 100 percent does not necessarily imply a specific health hazard, but merely that a possible risk cannot be ruled out with the required degree of certainty. No statement can be made about the probability of a health hazard. Particularly at-risk population groups are used to determine the degree of exhaustion, in this case two- to three-year-old children with an average body weight of 15 kilograms. [8] The weight figures for children in this age group are based on inquiries made to the Ministry for Climate Protection, Environment, Agriculture, Nature Conservation and Consumer Protection of the State of North Rhine-Westphalia, Nature and Consumer Protection of the State of North Rhine-Westphalia to WHO values for girls. [9] This takes into account the less favorable ratio of food intake to body weight. However, it is incomprehensible why an average body weight of 16.15 kilograms for two to five-year-olds is used as the basis for the assessment of pesticide residues [10].
Protein supplements not suitable for young children
The reference to children as a particularly vulnerable population group can generally be accepted. However, the target group for protein products is adults who take protein supplements due to special diets or sporting activity. Furthermore, consumption recommendations according to the Guideline Daily Amount (GDA) refer to an average adult with an energy requirement of 8,400 kilojoules / 2,000 kilocalories. Reference values for children are generally only found on products specially developed for children. [11] The administration of protein supplements to small children without medical indication is not recommended without further ado. 25 grams of hemp protein powder (minimum recommended intake for the average adult) corresponds to 12.5 grams of protein with a protein content of 50 percent. The German Nutrition Society (DGE) recommends a daily protein intake of one gram per kilogram of body weight for one to four-year-olds. [12] The values of the WHO [13] and Dietary Reference Intakes (DRI) [14] are even lower:
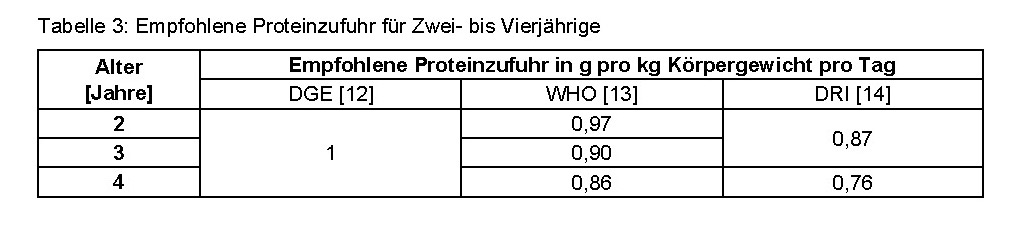
This would almost exhaust the recommended daily amount for a body weight of 15 kilograms. However, with sufficient energy intake and a balanced diet, the protein requirement is usually covered by food and supplementation carries the risk of overdosing, which can put a strain on the body, for example the kidneys, in the long term if there is insufficient fluid intake. If medically necessary, products should therefore be used that are tailored to the nutritional requirements of children.
Even in the case of vegan diets for children and adolescents, which are not recommended by the DGE in its position on vegan nutrition, qualified advice and regular monitoring of the supply of potentially critical nutrients such as essential amino acids by doctors or nutritionists is necessary in order to be able to take corrective action if necessary. For people with special nutritional requirements, such as vegan infants, specific food selection and preparation (high-quality, nutrient-dense foods, age-appropriate preparation and presentation) is required. [15] No allergenic potential compared to other protein sources
The simplistic suggestion made in reports that consumers should generally choose protein sources from nuts or soy cannot be followed without reservation. These foods have a very high allergenic potential. Although hemp seeds (raw material for hemp protein powder) are also classified as nuts from a botanical point of view, they have no allergenic potential.
The problem of a possibly excessive protein intake of small children also exists with all protein products used for supplementation, regardless of the main component used (soy, nut, milk protein).
Recommendation for adults not transferable to children
The different density of CB-1 receptors (cannabinoid receptors mainly in the central nervous system) as part of the endogenous cannabinoid system in children and adults is not taken into account in the guideline value analysis. [16] The receptors play a role in the coordination of movements, the processing of sensory impressions, pain processing and memory. [17] By docking to these receptors, delta-9-THC unfolds its psychoactive and therefore undesirable effect in foods.
Clinical studies have shown that young children are significantly less sensitive to delta-9-THC and tolerate a higher ratio of THC to body weight than adults. This may be due to the lower density of CB1 receptors. [2] It should therefore be noted that the guideline value of 1 microgram of THC for adults is not easily transferable to small children.
Conclusion
In principle, the administration of protein supplements to two- to three-year-old children without medical indication is not recommended without further ado. It is right to take food off the market as a precautionary measure if there is a potential risk, but without leaving the factual level in the public presentation..
The discussion of the THC issue should be conducted with care and the risk assessment should be based on valid information and facts so as not to compare facts that are not comparable. A reasonable assessment of the risks of hemp-containing foods based on valid data that takes all the different aspects into account is desirable. Current recommendations only reflect snapshots of the current results. The 2015 report by the nova-Institute and the EIHA provides scientifically sound proposals for new guideline value recommendations and a realistic assessment of the THC issue.
[2] Contact:
Hempro International GmbH & Co. KG
Münsterstr. 336
40470 Düsseldorf
Phone: 0211-699 90 56-10
Fax: 0211-699 90 56-18
E-mail: a.benske@hempro.com
Contact person:
Daniel Kruse (Management)
Andrea Benske (Quality Management)
This post was translated from DeepL.

